KIDNEY STONE DISEASES: causes,risk factors and possible cure.
INTRODUCTION
Kidney stone disease, also known as urolithiasis, is when a solid piece of material (kidney stone) occurs in the urinary tract. Kidney stones typically form in the kidney and leave the body in the urine stream. A small stone may pass without causing symptoms. If a stone grows to more than 5 millimeters (0.2 in) it can cause blockage of the ureter resulting in severe pain in the lower back or abdomen.
A stone may also result in blood in the urine, vomiting, or painful urination. About half of people will have another stone within ten years.
Most stones form due to a combination of genetics and environmental factors. Risk factors include high urine calcium levels, obesity, certain foods, some medications, calcium supplements, hyperparathyroidism, gout and not drinking enough fluids.
Stones form in the kidney when minerals in urine are at high concentration. The diagnosis is usually based on symptoms, urine testing, and medical imaging. Blood tests may also be useful.
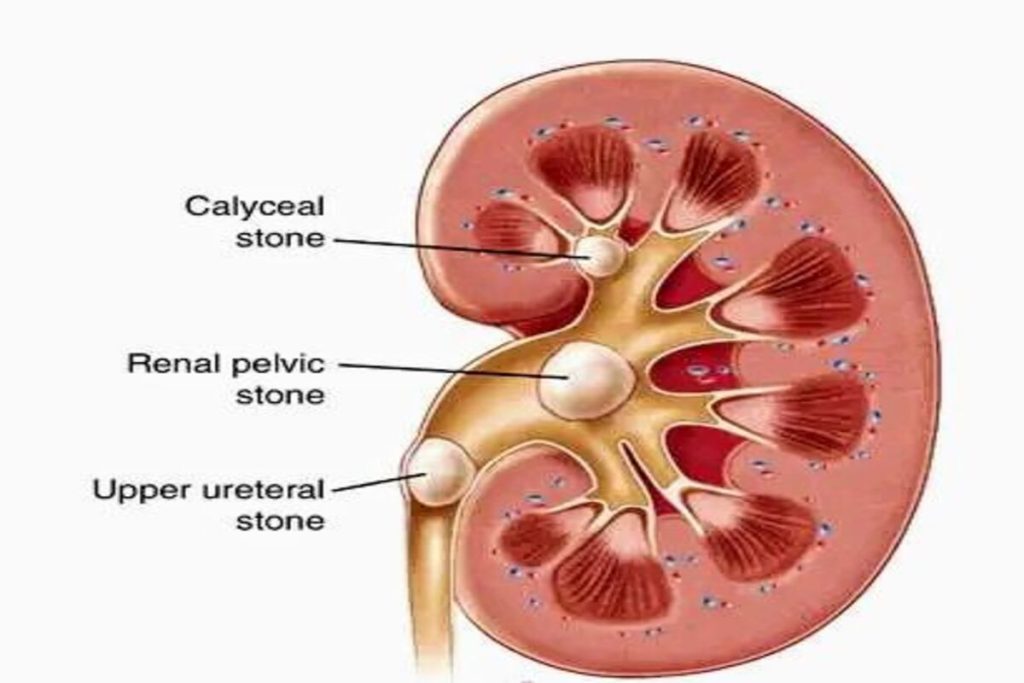
Stones are typically classified by their location:
nephrolithiasis (in the kidney)
ureterolithiasis (in the ureter)
cystolithiasis (in the bladder), or by what they are made of (calcium oxalate, uric acid, struvite, cystine).
Common preventive measures.
In those who have had stones, prevention is by drinking fluids such that more than two liters of urine are produced per day. If this is not effective enough, thiazide diuretic, citrate, or allopurinol may be taken. It is recommended that soft drinks containing phosphoric acid (typically colas) be avoided. When a stone causes no symptoms, no treatment is needed.
Otherwise pain control is usually the first measure, using medications such as nonsteroidal anti-inflammatory drugs or opioids. Larger stones may be helped to pass with the medication tamsulosin or may require procedures such as extracorporeal shock wave lithotripsy, ureteroscopy, or percutaneous nephrolithotomy.
Between 1% and 15% of people globally are affected by kidney stones at some point in their life. In 2015, 22.1 million cases occurred, resulting in about 16,100 deaths. They have become more common in the Western world since the 1970s.Generally, more men are affected than women.Kidney stones have affected humans throughout history with descriptions of surgery to remove them dating from as early as 600 BC.
SIGNS AND SYMPTOMS
The hallmark of a stone that obstructs the ureter or renal pelvis is excruciating, intermittent pain that radiates from the flank to the groin or to the inner thigh. This pain, known as renal colic, is often described as one of the strongest pain sensations known. Renal colic caused by kidney stones is commonly accompanied by urinary urgency, restlessness, hematuria, sweating, nausea, and vomiting. It typically comes in waves lasting 20 to 60 minutes caused by peristaltic contractions of the ureter as it attempts to expel the stone.
The embryological link between the urinary tract, the genital system, and the gastrointestinal tract is the basis of the radiation of pain to the gonads, as well as the nausea and vomiting that are also common in urolithiasis. Postrenal azotemia and hydronephrosis can be observed following the obstruction of urine flow through one or both ureters.
Pain in the lower-left quadrant can sometimes be confused with diverticulitis because the sigmoid colon overlaps the ureter, and the exact location of the pain may be difficult to isolate due to the close proximity of these two structures.
RISKS FACTORS
Dehydration from low fluid intake is a major factor in stone formation.
Obesity is a leading risk factor as well.
High dietary intake of animal protein, sodium, sugars including honey, refined sugars, fructose and high fructose corn syrup,oxalate,grapefruit juice, and apple juice may increase the risk of kidney stone formation.
Kidney stones can result from an underlying metabolic condition, such as distal renal tubular acidosis, Dent’s disease, hyperparathyroidism, primary hyperoxaluria,or medullary sponge kidney. 3–20% of people who form kidney stones have medullary sponge kidney.
Kidney stones are more common in people with Crohn’s disease; Crohn’s disease is associated with hyperoxaluria and malabsorption of magnesium.
A person with recurrent kidney stones may be screened for such disorders. This is typically done with a 24-hour urine collection. The urine is analyzed for features that promote stone formation.
SURGERY
Most stones under 5 mm (0.2 in) pass spontaneously. Prompt surgery may, nonetheless, be required in persons with only one working kidney, bilateral obstructing stones, a urinary tract infection and thus, it is presumed, an infected kidney, or intractable pain. Beginning in the mid-1980s, less invasive treatments such as extracorporeal shock wave lithotripsy, ureteroscopy, and percutaneous nephrolithotomy began to replace open surgery as the modalities of choice for the surgical management of urolithiasis. More recently, flexible ureteroscopy has been adapted to facilitate retrograde nephrostomy creation for percutaneous nephrolithotomy. This approach is still under investigation, though early results are favorable. Percutaneous nephrolithotomy or, rarely, anatrophic nephrolithotomy, is the treatment of choice for large or complicated stones (such as calyceal staghorn calculi) or stones that cannot be extracted using less invasive procedures.
OPEN PYELOLITHOTOMY
INTRODUCTION
Pyelolithotomy is an operation that is now uncommonly performed. The advent of percutaneous nephrolithotomy with contact lithotripsy (PCN) and extracorporeal shockwave lithotripsy (ESWL) has reduced the indications for pyelolithotomy, which is a considerably more invasive procedure.
It is interesting to note that when surgeons were first performing pyelolithotomy, there was considerable disagreement as to which was the preferred approach to stone removal. Clearly, people liked to argue even then, when one had to do so mainly by letter or book rather than by published article, conference, phone call, telefax, or Internet. Vincenz Czerny probably performed the first pyelotomy, with Sir Henry Morris performing a similar operation to remove a stone in the same year, 1880.
Further developments took place over the years, with many incisions through the thorax and abdomen being introduced, and then many incisions through the renal pelvis and renal parenchyma following. The introduction of radiologic visualization of the kidney completed the picture. It is clear, however, that the landmark contributions to open surgical removal of stones from the kidney were made in recent years by Gil Vernet, Marshall, Boyce, and Wickham.
DIAGNOSIS
The usual presenting symptom for renal calculi is radiating colicky flank pain, usually associated with hematuria. Larger stones, however, may be relatively asymptomatic or present with persistent infection and/or hematuria. The diagnosis of renal calculi is generally made radiographically. Currently, the most common radiologic method of diagnosis is via a KUB and intravenous pyelography, though some centers are investigating the use of ultrasound and computed tomography.
SURGICAL TECHNIQUE
Surgical Access to the Kidney
The urologist may consider five possible approaches to the kidney for open stone removal:
1.Flank approach
a. Subcostal
b.Costal (11th or 12th rib)
c.Intercostal (above the 11th or 12th rib)
2.Transabdominal
3.Posterior lumbotomy
The advantages of the flank approach are described after I explain why, in my opinion, the transabdominal and posterior lumbotomy incisions are rarely required in what is, after all, a relatively uncommon procedure. Transabdominal and transperitoneal access may be required if the patient has spinal deformities and very occasionally after several previous surgical procedures. It is the most invasive of all the approaches, and recovery is delayed postoperatively. It should not be used as the standard approach for pyelolithotomy.
The posterior lumbotomy incision has its advocates, particularly because postoperative pain is minimal, and recovery is quick with shortened hospital stay. The patient is placed either in the lateral decubitus position or prone, with pillows under the upper abdomen. The incision is made about 2.5 cm lateral to the erector spinae muscle from the 12th rib down to the superior border of the iliac crest. The incision is extended through fat and fascia and then through the aponeurotic fibers of the latissimus dorsi. If further access is required, a small part of the 12th rib can be removed, or the lower end of the incision can be curved inferolaterally along the iliac crest. The advantages of this approach have been listed above, but a major disadvantage is that access to the upper pole is difficult, as is access to the ureter below its upper portion. I feel that when an open operation is being performed today for stone removal, it is unlikely to be a simple procedure but rather a more complex one for which greater exposure may be required than is afforded by this incision.
Of the flank incisions, I prefer the costal route of access. The subcostal approach is usually too low for renal surgery of any complexity. In considering the incision, it is worth remembering that although it is possible to be too low, preventing complete visualization of each step of the subsequent dissection, it is never possible to be too high. For this reason, the skin incision should be made on top of or superior to one of the ribs. In this way, damage to the subcostal or infracostal nerves is prevented, and the likelihood of a wound hernia is minimized. The intercostal approach requires division of the posterior costotransverse ligament in order to allow the rib to “bucket-handle.” Otherwise, access between the ribs may be suboptimal, and, indeed, the ribs may break when spread apart by a self-retaining retractor.
When an incision is based on a rib (the costal approach), removal of the end of the rib is required. A careful review of the preoperative x-ray films and examination of the patient with the table broken will clarify whether the 12th, 11th, or even, on some occasions, the tenth rib should be the line of the skin incision. The patient should be placed on the table in the lateral decubitus position with the side to be operated on facing directly upward. The patient can be stabilized by inserting three T-pieces along the sides of the table or by fastening tape to the upper thorax and over the hip, thereby fixing the patient on the table. The table is broken, thus opening up the space between the ribs and the iliac crest, and then tilted 20 degrees laterally toward the surgeon. The surgeon and assistant are both positioned behind the patient, and the surgeon can sit down throughout the procedure.
The incision is made in the skin over the distal 6 cm of the rib, extending medially for another 10 to 12 cm. It should be deepened down to the rib before any muscles are cut. Once the rib can be clearly seen, the skin, fat, and fascial layers are retracted on both sides to give a better view. The intercostal muscles are divided above the rib with a knife until the diaphragm can be seen; this is then divided with a scissors until the distal 6 cm of rib is cleared. Then the same approach is made below the rib, with the intercostal muscles being divided if the 11th rib is being used, or the latissimus dorsi if it is the 12th. The diaphragm will not be divided inferior to the rib, but it is advisable to identify the nerve bundle and sweep it inferiorly. Once the rib has been dissected free of the surrounding muscles, the distal 6 cm is removed with a rib shears. I do not approach the rib subperiosteally, as leaving the periosteum does not confer any advantage.
Once the rib has been removed, Gerota’s fascia can be visualized, and through it the kidney can be palpated. Two fingers should be introduced under the abdominal muscles through this incision, and the peritoneum swept away from their under surface. The incision is then deepened through the external oblique, internal oblique, and transversus abdominus muscles using the knife or cutting diathermy. The incision should not extend as far medially as the rectus sheath.
At this stage, a body wall retractor should be inserted, preferably the Wickham retractor, which is self-retaining and shaped to the body wall. Gerota’s fascia is then opened, and this incision is extended upward toward the diaphragm and inferiorly toward the pelvic brim. The peritoneum can then be mobilized medially away from the ureter, which is visualized exiting from the perirenal fat, and the ureter is encircled with a loop or tape. The perirenal fat is then grasped over the lateral border of the kidney, elevated by two Babcock forceps, and incised, revealing the capsule of the kidney. The degree of mobilization of the kidney required depends on how large the stone is. If full mobilization is required, it can be performed easily but should always be carried out by sharp dissection with a Metzenbaum scissors under direct vision. Remember that the main renal vein is always best accessed from anterior to the kidney (although there may be tributaries lying posteriorly). The main renal artery is best approached from above and posterior to the kidney, although there may be other branches, particularly an upper pole or lower pole branch directly from the aorta. The artery need not be isolated in the unusual situation that small stones are being removed, unless a parenchymal incision is being contemplated. When it is isolated, a loop or tape should be put around it.
In the case of surgical access after previous surgery and after many previous surgical procedures, great care must be taken. After incision of the muscles, the fascial and perirenal areas are likely to be greatly thickened and indurated. It is very helpful to isolate the ureter first (prestented if required) and to trace the ureter upward to the ureteropelvic junction. The kidney can then be dissected free from the surrounding tissues with the scissors. Care must be taken not to incise the renal capsule because considerable hemorrhage can occur under these circumstances. Because the kidney is likely to be encased in dense fibrous tissue, the perirenal anatomy will be difficult to define accurately, and upper and lower pole arteries can be damaged. In addition, identification of the renal artery can be somewhat more difficult; palpation of the tissues medial to the kidney will reveal its position.
COMPLICATIONS
Complications from open renal stone surgery are significant and include hemorrhage, urinary fistula, recurrent stones, and actual or functional loss of the renal unit. The risk of these complications is dependent on the associated findings of chronic infection, prior surgery, and surgeon’s expertise.
There is a small chance that the pleura may be opened when a costal or intercostal incision is made, and the probability of this increases the higher the incision is made. A pleurotomy is readily identified by hearing the sound of air being sucked into the thorax and by seeing the lung on inspiration. The diaphragm should be dissected free from the ribs and used to strengthen the closure of the pleura, which is itself too thin and fragile to hold a suture. The 3-0 chromic catgut suture should include the diaphragm, pleura, and intercostal muscles, and the anesthesiologist should inflate the lung before the last suture is put in. This usually prevents a pneumothorax. A postoperative chest x-ray must be performed, and, in the relatively uncommon event of a persistent pneumothorax, a chest tube should be inserted.